Guilty Plea: Lab Owner Falsified COVID Test Results During Pandemic

Table of Contents
The Scale of the Fraud and its Impact
The sheer scale of this fraudulent activity is alarming. The lab owner, [Name of Lab Owner, if available, otherwise remove this part], pleaded guilty to falsifying an estimated [Number] COVID-19 test results. This staggering number significantly impacted public health efforts across [Geographic Area impacted, e.g., multiple states, a specific county].
Number of Falsified Test Results
While the exact number remains under investigation, authorities estimate that [Number] or more tests were falsified. This uncertainty itself underscores the gravity of the situation, as the true extent of the impact may be even greater than currently understood. The lack of accurate data hindered effective response and created a ripple effect of consequences.
Geographic Impact
The fraudulent activities primarily affected [Geographic Area, e.g., several counties in California, the entire state of Texas]. This geographically concentrated impact, however, doesn't diminish the potential for widespread consequences, as infected individuals could have easily traveled to other regions, unknowingly spreading the virus.
- Impact on contact tracing efforts: Inaccurate results severely hampered contact tracing efforts, hindering the ability of public health officials to identify and isolate infected individuals, potentially leading to further outbreaks.
- Potential spread of the virus: False negative results allowed infected individuals to believe they were healthy, leading to continued social interaction and increased transmission rates. This amplified the spread of the virus and prolonged the pandemic's impact.
- Economic impact: Individuals and businesses suffered due to inaccurate results leading to unnecessary quarantines and economic losses. False positives caused unnecessary stress and disruption, while false negatives hampered effective containment strategies.
- Erosion of public trust: This fraudulent activity significantly eroded public trust in testing facilities and healthcare systems in general, further complicating pandemic response efforts.
The Charges and the Plea Agreement
The lab owner faced several serious charges, including [Specific Charges, e.g., healthcare fraud, wire fraud, obstruction of justice]. These charges reflect the severity of the actions and the potential harm inflicted upon the public.
Specific Charges Filed Against the Lab Owner
The indictment detailed charges under [Specific Laws Violated, e.g., 18 U.S. Code § 1341 (Mail Fraud), 18 U.S. Code § 1343 (Wire Fraud)]. The prosecution highlighted the deliberate nature of the fraud, demonstrating a pattern of falsifying results for financial gain.
Terms of the Plea Agreement
The plea agreement resulted in a guilty plea to [Specific Charge(s) from Plea]. The lab owner faces a potential sentence of [Sentence Length or Range], along with significant fines and restitution to affected individuals and organizations.
- Laws Violated: The charges involved violating multiple federal statutes designed to protect public health and prevent fraudulent activities in healthcare settings.
- Cooperation with Investigators: The plea agreement may have included a clause requiring the lab owner to cooperate fully with ongoing investigations, potentially leading to the identification of other individuals involved.
- Consequences for Lab Operation: The lab's license has been revoked, and its operations have been permanently shut down.
The Investigation and its Revelations
The fraudulent activities were initially uncovered through [Method of Discovery, e.g., a whistleblower report, an internal audit that revealed discrepancies in data]. This discovery triggered a thorough investigation by [Regulatory Agencies Involved, e.g., the FBI, state health departments].
How the Fraud Was Uncovered
Discrepancies in reported COVID-19 case numbers and inconsistencies within the lab's own data initially raised suspicion. Further investigation revealed a pattern of falsified results.
Role of Regulatory Agencies
The [Regulatory Agencies] played a crucial role in the investigation, coordinating efforts to gather evidence and build a strong case against the lab owner. Their response highlights the importance of regulatory oversight in maintaining the integrity of the healthcare system.
- Timeline of the Investigation: The investigation spanned [Time Period], from the initial discovery to the guilty plea.
- Techniques Used to Detect Fraud: Investigators utilized data analysis techniques, cross-referencing data from various sources to pinpoint the fraudulent activity.
- Other Individuals Implicated: The investigation is ongoing, and further indictments may be forthcoming.
Prevention and Future Implications
This case underscores the need for significant changes to prevent future occurrences of falsified COVID-19 test results and similar healthcare fraud.
Increased Oversight and Regulation
Increased regulatory oversight and stricter penalties for fraudulent activities are crucial. This includes more frequent audits of testing facilities, stricter licensing requirements, and enhanced monitoring of data integrity.
Improving Data Integrity in Testing
Implementing robust data validation systems and enhanced auditing protocols can help prevent future incidents. This requires investment in technology and a commitment to rigorous quality control measures.
- Improved Data Validation Systems: Real-time data verification systems can flag inconsistencies and potential errors, alerting authorities to suspicious activity.
- Enhanced Auditing Protocols: Regular, independent audits should be mandatory for all testing facilities, including rigorous checks on data integrity and compliance with regulations.
- Strengthened Penalties for Fraudulent Activity: Significantly harsher penalties for fraudulent activity will serve as a strong deterrent.
Conclusion: Learning from the Guilty Plea: Falsified COVID Test Results
This case of "Guilty Plea: Lab Owner Falsified COVID Test Results During Pandemic" serves as a sobering reminder of the devastating consequences of dishonesty and fraud within the healthcare system. The scale of the fraudulent activity, its impact on public health, and the erosion of public trust are deeply concerning. Accountability and transparency are paramount in maintaining the integrity of our healthcare system and ensuring public safety.
We must learn from this incident and implement effective measures to prevent similar occurrences in the future. If you suspect fraudulent activity related to COVID-19 testing or other healthcare services, please report it immediately to [Link to relevant reporting agency 1] or [Link to relevant reporting agency 2]. Reporting suspicious activity related to falsified COVID test results, fraudulent COVID testing, or any form of COVID-19 testing fraud is crucial to protecting public health.

Featured Posts
-
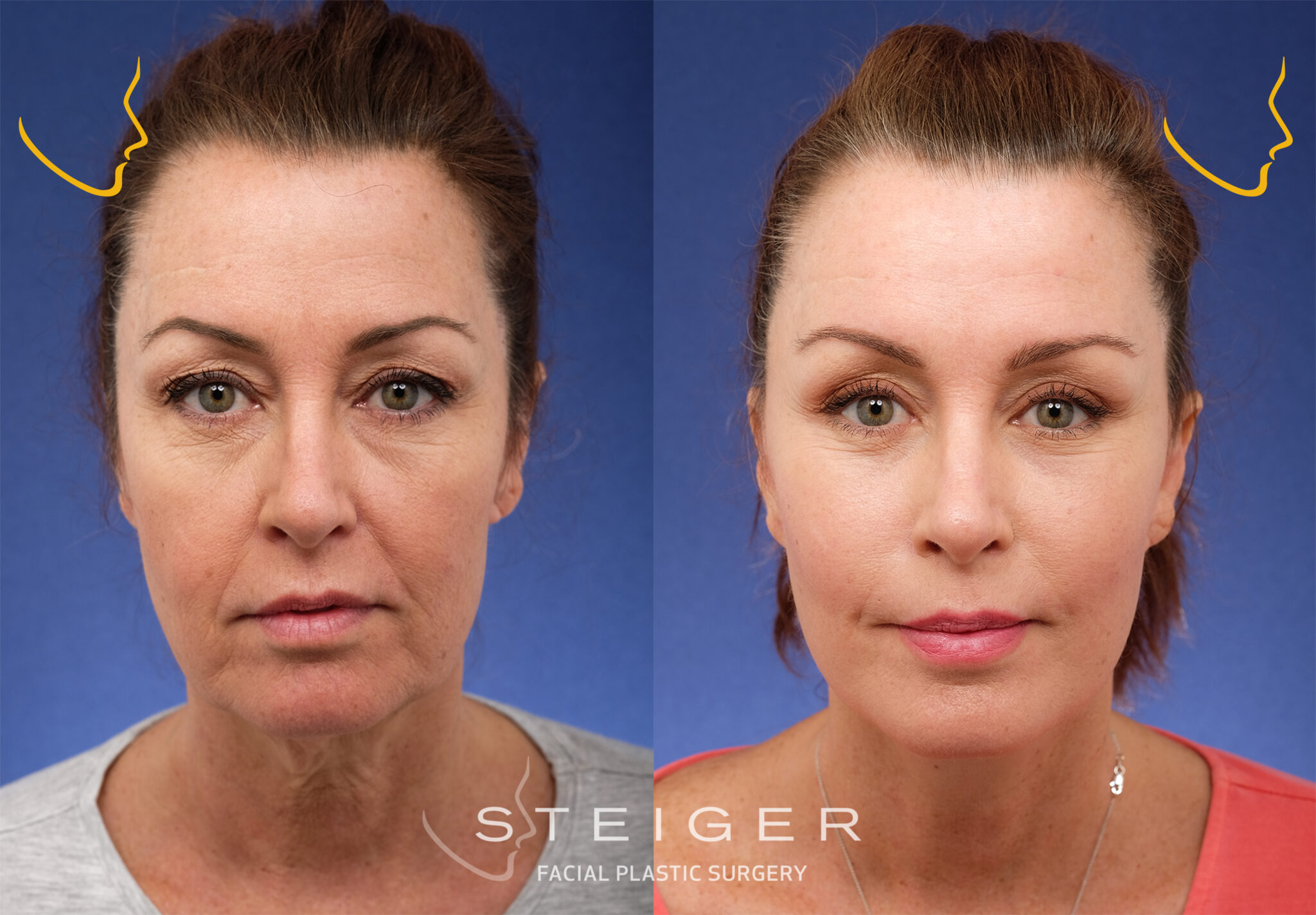 Dramatic Facelift Leaves Fans Questioning Stars New Look
May 03, 2025
Dramatic Facelift Leaves Fans Questioning Stars New Look
May 03, 2025 -
 Fortnite Update Problems Chapter 6 Season 2 Launch Postponed Due To Extended Downtime
May 03, 2025
Fortnite Update Problems Chapter 6 Season 2 Launch Postponed Due To Extended Downtime
May 03, 2025 -
 4 G Wh Bess Project In Netherlands Lion Storage Announces Financial Close
May 03, 2025
4 G Wh Bess Project In Netherlands Lion Storage Announces Financial Close
May 03, 2025 -
 Christina Aguileras Photoshopped Photos Fans React To Unrecognizable Images
May 03, 2025
Christina Aguileras Photoshopped Photos Fans React To Unrecognizable Images
May 03, 2025 -
 Green Transportation Exploring The Potential Of Wind Powered Trains
May 03, 2025
Green Transportation Exploring The Potential Of Wind Powered Trains
May 03, 2025
