China Seeks Domestic Alternatives To US Pharmaceuticals

Table of Contents
Government Policies and Initiatives Driving Domestic Pharmaceutical Production
The Chinese government has launched a series of ambitious policies and initiatives to accelerate the growth of its domestic pharmaceutical industry. These efforts are aimed at reducing reliance on foreign drug imports and fostering self-sufficiency in essential medicines. Key strategies include substantial financial incentives, streamlined regulatory processes, and targeted support for promising domestic companies.
-
Substantial Financial Incentives: The government provides generous subsidies, tax breaks, and preferential loan schemes to domestic pharmaceutical manufacturers, stimulating investment in production capacity and technological upgrades. These incentives are particularly focused on companies producing essential medicines and those engaged in innovative drug development.
-
Streamlined Regulatory Approvals: China has implemented reforms to simplify and expedite the approval process for new drugs and generic medications developed domestically. This faster approval process enables domestic companies to bring their products to market more quickly, gaining a competitive edge.
-
Specific Government Programs: Initiatives like the "Made in China 2025" plan and various national health initiatives directly support the domestic pharmaceutical sector. These programs provide funding, technical assistance, and strategic guidance to boost production and R&D capabilities.
-
Examples of Success: Companies like CSPC Pharmaceutical Group and Huadong Medicine have emerged as leading domestic players, contributing significantly to the growth of the sector and showcasing the effectiveness of government support. Their successes illustrate the potential for domestic pharmaceutical companies to compete effectively on the global stage.
Specific Policy Changes:
- Relaxation of regulations for generic drug approvals.
- Increased funding for research and development of domestically produced APIs (Active Pharmaceutical Ingredients).
- Establishment of specialized industrial parks focused on pharmaceutical manufacturing.
Statistics: The growth of the domestic pharmaceutical market in China is substantial, with year-on-year growth consistently exceeding global averages in recent years. (Specific data should be inserted here from reliable sources such as government reports or industry analyses).
Investing in Research and Development (R&D) for Innovative Drugs
To truly achieve pharmaceutical independence, China recognizes the critical need to develop its own innovative drugs, rather than simply relying on generic versions of foreign pharmaceuticals. Significant investments are being channeled into R&D, fostering collaborations between universities, research institutions, and the private sector.
-
Collaborative R&D: The government actively encourages partnerships between academic institutions, research centers (like those under the Chinese Academy of Sciences), and pharmaceutical companies. These collaborations combine scientific expertise with industrial capabilities, accelerating the drug development pipeline.
-
Government Funding: Billions of yuan are allocated annually to pharmaceutical R&D through various government grants, research programs, and funding initiatives. This substantial funding stream fuels innovation and the development of cutting-edge technologies.
-
Focus Areas: Research efforts are concentrated on areas of unmet medical needs, particularly in chronic diseases prevalent in China's aging population, such as diabetes, cardiovascular diseases, and cancer.
Examples of Successful R&D Collaborations: (Insert examples of successful collaborations and resulting drug discoveries here).
Challenges in R&D:
- Attracting and retaining top scientific talent within China.
- Protecting intellectual property rights and preventing the theft of research findings.
- Navigating complex regulatory hurdles and ensuring timely clinical trials.
Strengthening the Domestic Supply Chain and Manufacturing Capabilities
A robust and reliable domestic supply chain is essential for China's pharmaceutical independence. This involves enhancing manufacturing infrastructure, securing access to raw materials, and ensuring stringent quality control measures.
-
Advanced Manufacturing Facilities: Significant investments are being made in constructing and upgrading pharmaceutical manufacturing facilities, adopting advanced technologies to improve efficiency and quality. This includes investments in automation, precision manufacturing, and sterile production environments.
-
Domestic API Production: China is actively promoting the domestic production of APIs, which are the fundamental building blocks of many pharmaceuticals. Reducing reliance on foreign API suppliers is a key goal to ensure supply chain security.
-
Quality Control: Strict adherence to international quality standards (like GMP – Good Manufacturing Practices) is being enforced throughout the pharmaceutical supply chain. This ensures the safety and efficacy of domestically produced drugs, both for the domestic market and for potential exports.
Investments in Infrastructure: (Insert details about specific investments in manufacturing facilities and related infrastructure).
Challenges and Obstacles in Achieving Pharmaceutical Independence
Despite significant progress, China faces considerable challenges in its quest for pharmaceutical self-reliance.
-
Global Competition: Competing with established multinational pharmaceutical companies, possessing vast resources and experience, presents a formidable challenge for Chinese companies.
-
Talent Acquisition and Retention: Attracting and retaining highly skilled scientists, researchers, and pharmaceutical professionals is crucial for innovation and growth. Competition for talent with other sectors and international organizations is intense.
-
Intellectual Property Protection: Protecting intellectual property rights is vital to incentivize innovation. Concerns remain about the enforcement of intellectual property laws and preventing biosimilar development that infringes on patents.
-
Maintaining International Standards: Meeting and exceeding international quality and safety standards is paramount to gain global recognition and market access for domestically produced pharmaceuticals.
Specific Examples of Challenges: (Insert specific examples of challenges faced by domestic companies, such as difficulties in securing funding, navigating regulatory processes, or competing with established global players).
Conclusion: The Future of China's Pharmaceutical Landscape
China's drive to reduce its dependence on US pharmaceuticals is a complex and long-term undertaking. The strategies being implemented – government incentives, substantial investments in R&D, strengthening of the domestic supply chain, and a focus on quality control – represent a significant commitment to national health security and economic independence. However, achieving true pharmaceutical self-reliance requires overcoming significant challenges related to global competition, talent acquisition, intellectual property protection, and maintaining international standards.
Stay informed about the progress of China's efforts to achieve pharmaceutical independence and the implications for global healthcare. Continue following the developments in China's pursuit of domestic alternatives to US pharmaceuticals.

Featured Posts
-
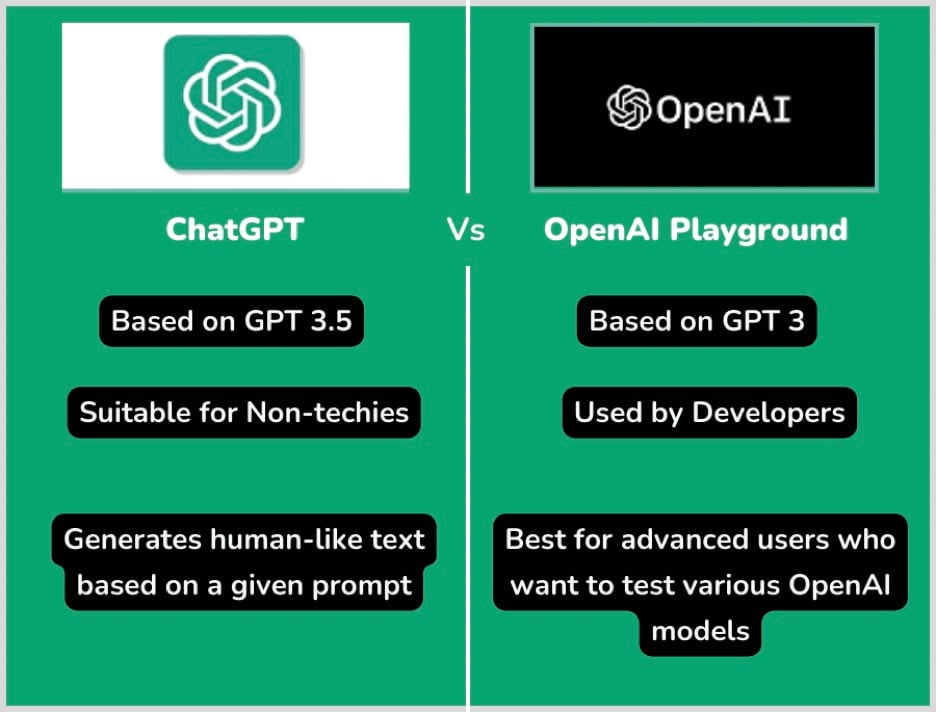 Chat Gpt Vs Google Shopping Open Ais Latest Challenge
May 01, 2025
Chat Gpt Vs Google Shopping Open Ais Latest Challenge
May 01, 2025 -
 Investigation Into Multi Million Dollar Nfl Heists Chilean Migrants Charged
May 01, 2025
Investigation Into Multi Million Dollar Nfl Heists Chilean Migrants Charged
May 01, 2025 -
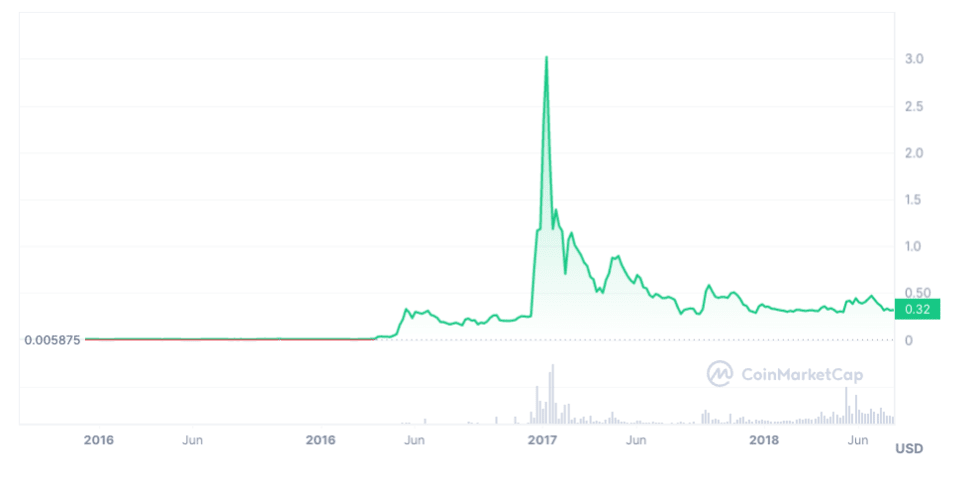 Xrp Future Price Analyzing The Post Sec Lawsuit Market
May 01, 2025
Xrp Future Price Analyzing The Post Sec Lawsuit Market
May 01, 2025 -
 Buy Xrp Ripple Now A Deep Dive Into The Sub 3 Price
May 01, 2025
Buy Xrp Ripple Now A Deep Dive Into The Sub 3 Price
May 01, 2025 -
 Tragedy Strikes After School Camp Car Crash Results In Four Deaths
May 01, 2025
Tragedy Strikes After School Camp Car Crash Results In Four Deaths
May 01, 2025
Latest Posts
-
 19 2025
May 01, 2025
19 2025
May 01, 2025 -
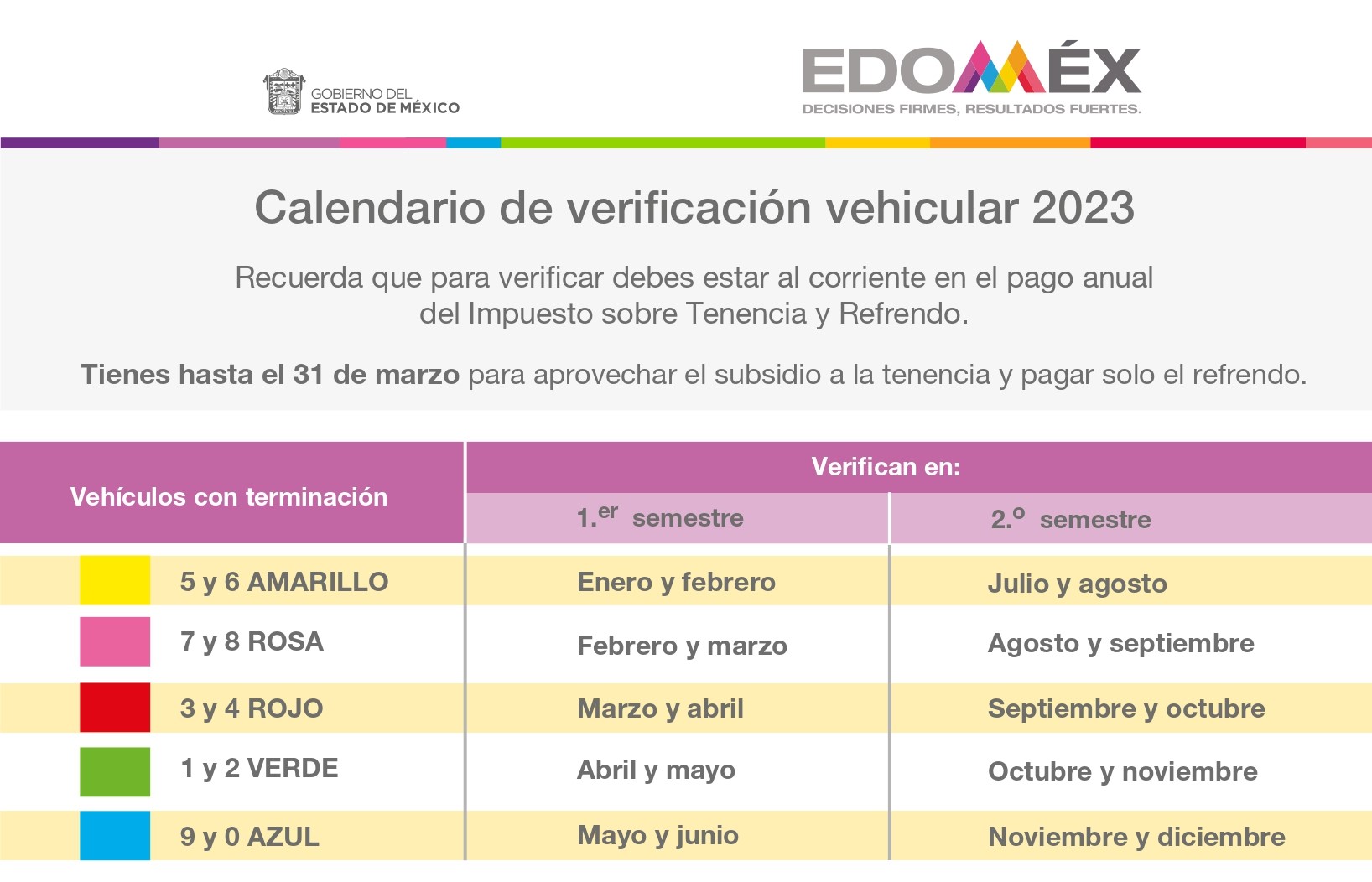 Clases De Boxeo En Edomex Inscripcion Cierra En 3 Dias
May 01, 2025
Clases De Boxeo En Edomex Inscripcion Cierra En 3 Dias
May 01, 2025 -
 No Te Quedes Fuera Clases De Boxeo Edomex 3 Dias
May 01, 2025
No Te Quedes Fuera Clases De Boxeo Edomex 3 Dias
May 01, 2025 -
 Clases De Boxeo Edomex Apurate Solo 3 Dias
May 01, 2025
Clases De Boxeo Edomex Apurate Solo 3 Dias
May 01, 2025 -
 Inscripciones De Boxeo Edomex 3 Dias Restantes
May 01, 2025
Inscripciones De Boxeo Edomex 3 Dias Restantes
May 01, 2025
